8 Things to Know about the Next Evolution of Meaningful Use
8 Things to Know about the Next Evolution of...
“Top 10” List for Security Law Compliance
“Top 10” List for Security Law Compliance As we...
CMS Extends Hospital Deadline for Meaningful Use Attestation
CMS Extends Hospital Deadline for Meaningful Use...
CMS Releases Final Meaningful Use CEHRT Extension Rule
CMS Releases Final Meaningful Use CEHRT...
SAMHSA Public Session to Discuss Part 2 Regulations & HIE
SAMHSA Public Session to Discuss Part 2...
Reminder: Public Comment Period Open for Meaningful Use NPRM
Reminder: Public Comment Period Open for...
When Do Conduits Cross the HIPAA BA Line?
What Is a “Conduit” and When Do They Cross The...
CMS Announces Notice of Proposed Rulemaking for 2014 Certified EHR Technology Flexibility
CMS Announces Notice of Proposed Rulemaking for...
Webinar: HIPAA and HIT Best Practices for Hospital Executives & Board Trustees
Webinar: HIPAA and HIT Best Practices for...
OIG Finds Fault with CMS Meaningful Use Oversight
OIG Finds Fault with CMS Meaningful Use...
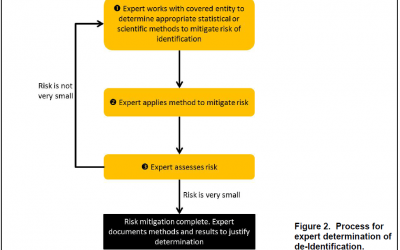
OCR Releases HIPAA De-identification Q&A Guidance
OCR Releases HIPAA De-identification Q&A...
SERCH Project Recommendations for HIE and Disaster Preparedness
SERCH Project Recommendations for HIE and...
Last Call for Hospital Attestations
Last Call for Hospital Attestations ...
What do Thanksgiving, HIE and Disaster Recovery Have in Common?
What do Thanksgiving, HIE and Disaster Recovery...
Guess What? OIG DOES Care about EHRs and Meaningful Use
Guess What? OIG DOES Care about EHRs and...
“Final Countdown” to the HITECH Omnibus Rule
“Final Countdown” to the HITECH Omnibus...
Is NwHIN going from “Free” to “For Fee”?
Is NwHIN going from “Free” to “For Fee”? Notable...
BCBS Plans Defend against Antitrust Class Actions
BCBS Plans Defend against Antitrust Class...
August Goes Out with a Bang: Stage 2 Final Rule & HIPAA Arrest
August Goes Out with a Bang: Stage 2 Final Rule...
CMS Meaningful Use Audits Begin; Stage 2 Rule Sent to OMB for Review
CMS Meaningful Use Audits Begin; Stage 2 Rule...
HHS Partners with Private Sector to Combat Healthcare Fraud
HHS Partners with Private Sector to Combat...
Subscribe & Survive the onslaught of new healthcare regulations requiring updates to affected compliance programs.
Get access to exclusive subscription-only access to resources, tools, industry analysis and other valuable solutions.